 Home
Home
 Back
Back
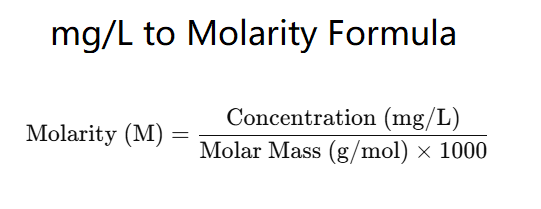
The formula for converting concentration from mg/L to molarity is:
\( \text{Molarity (M)} = \frac{\text{Concentration (mg/L)}}{\text{Molar Mass (g/mol)} \times 1000} \)
Let's say we have a solution with a concentration of 1000 mg/L, and the molar mass of the substance is 58.44 g/mol (like sodium chloride, NaCl). The molarity would be calculated as follows:
\( \text{Molarity} = \frac{1000 \, \text{mg/L}}{58.44 \, \text{g/mol} \times 1000} = 0.0172 \, \text{mol/L} \)
So the molarity of this solution would be 0.0172 mol/L.