 Home
Home
 Back
Back
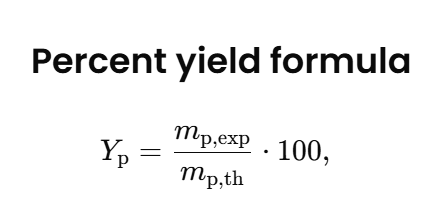
Definition: This calculator determines the percent yield of a chemical reaction based on the actual yield and theoretical yield, expressed as a percentage.
Purpose: It is used in chemistry to quantify the efficiency of a reaction, comparing the actual product obtained to the theoretical maximum, aiding in process optimization and analysis.
The calculator uses the percent yield formula:
Explanation: Enter the actual yield (e.g., 85 g) and theoretical yield (e.g., 100 g) with their respective units. The calculator converts units to grams, computes the percent yield, and displays it as a percentage.
Details: Percent yield measures reaction efficiency, indicating losses due to side reactions, incomplete reactions, or experimental errors, crucial for industrial and academic chemical processes.
Tips: Input the actual yield and theoretical yield with their units (e.g., mg, g, kg). Ensure both values are positive, and the theoretical yield is not zero to avoid division errors. The percent yield will be between 0% and 100% (or higher if experimental yield exceeds theoretical, indicating possible errors).