 Home
Home
 Back
Back
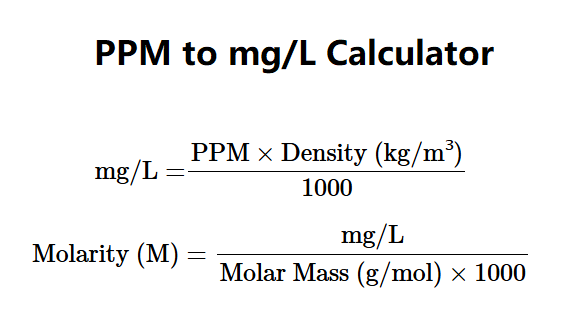
Definition: This converter transforms parts per million (ppm) into milligrams per liter (mg/L) using solute density.
Purpose: It is used in chemistry and environmental science to determine concentration levels in solutions.
Concentration in mg/L is calculated using:
Density conversions to kg/m³:
Explanation: Density is converted to kg/m³, then multiplied by ppm and divided by 1000 to get mg/L. Other units are derived from mg/L.
Details: Accurate conversion is critical for water quality analysis, chemical dosing, and environmental monitoring.
Tips: Enter the ppm value and solute density (in units like lb/ft³, g/mL, etc.; >0). Results include mg/L, µg/L, g/L, and kg/m³.