 Home
Home
 Back
Back
Definition: This converter transforms parts per million (PPM) into grams per liter (g/L), a unit of mass concentration in a solution.
Purpose: It is used in chemistry, environmental science, and pharmacology to measure the mass of a solute per liter of solution.
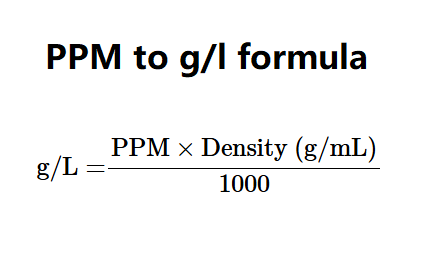
The conversion from PPM to g/L is calculated using:
Density conversions to g/mL:
Details: This conversion is essential for preparing solutions, analyzing water quality, and determining chemical dosages, especially in large volumes.
Tips: Enter the PPM value (>0) and optionally the solution density (default is 1.0 g/mL). Click "Convert" to get results in g/L, mg/L, and mg/kg.