 Home
Home
 Back
Back
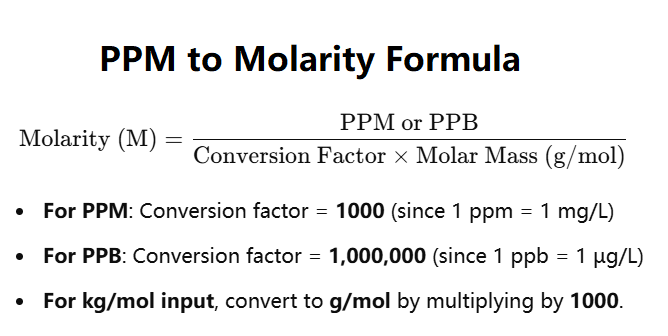
PPM (parts per million) and PPB (parts per billion) are concentration units often used in chemistry.
The formula to convert PPM/PPB to molarity is:
\(\text{Molarity} = \frac{\text{Concentration}}{\text{Conversion Factor} \times \text{Molar Mass}}\)
Example: You have **100 ppm** of NaCl in water. The molar mass of NaCl is **58.44 g/mol**.
Using the formula:
Molarity = \( \frac{100}{1000 \times 58.44} \) = 0.00171 M
So, **100 ppm NaCl ≈ 1.71 mM, 1710 µM, 1710000 nM**.