 Home
Home
 Back
Back
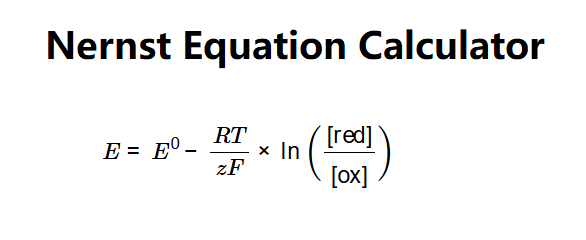
Definition: This calculator determines the reduction potential (\(E\)) of a half-cell or full-cell reaction using the Nernst equation, based on the standard reduction potential (\(E^0\)), temperature, number of moles of electrons transferred, and activities of the reduced and oxidized forms.
Purpose: It is used in electrochemistry to predict cell potentials under non-standard conditions, aiding in battery design, corrosion studies, and biochemical reaction analysis.
The calculator uses the Nernst equation:
Where:
Explanation: Enter the standard reduction potential (e.g., 0.34 V for Cu²⁺/Cu), temperature (e.g., 25°C), number of electrons transferred (e.g., 2 mol for Cu²⁺ + 2e⁻ → Cu), and activities of reduced and oxidized forms (e.g., 1 for both for standard conditions). The calculator converts units, computes \(E\) in volts, and displays the result.
Notes on Units: Ensure activities are positive and non-zero, temperature is above absolute zero, and \(z\) is a positive integer. Activities can be approximated by concentrations for simplicity, but exact activities are preferred for accuracy.
Details: The Nernst equation is crucial for understanding electrochemical cell potentials under non-standard conditions, predicting reaction spontaneity, and optimizing processes in batteries, fuel cells, and biological systems.
Tips: Input \(E^0\) in volts, temperature in °C, °F, or K, electrons transferred in moles, and activities (or concentrations) as positive numbers. Ensure \(z\) is an integer (e.g., 1, 2, 3) and activities are physically meaningful. Results are approximate for ideal solutions; adjust for non-ideal behavior if needed.