 Home
Home
 Back
Back
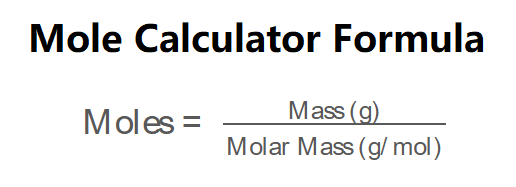
The formula for calculating moles from mass and molar mass is:
\( \text{Moles} = \frac{\text{Mass (g)}}{\text{Molar Mass (g/mol)}} \)
Let’s say you have 73 g of hydrochloric acid (HCl) with a molar mass of 36.5 g/mol. To find the moles:
\( \text{Moles} = \frac{73 \, \text{g}}{36.5 \, \text{g/mol}} = 2.0000 \, \text{mol} \)
This would be equivalent to:
2000.0000 mmol, 2000000.0000 µmol, 2000000000.0000 nmol, 2000000000000.0000 pmol.
A mole is the amount of substance that contains exactly 6.02214076 × 10²³ particles (Avogadro’s number), such as atoms, molecules, or ions. It’s used to measure large quantities of very small entities in chemical reactions.
Use the formula \( \text{Moles} = \frac{\text{Mass (g)}}{\text{Molar Mass (g/mol)}} \). Input the mass (in grams) and molar mass (in grams per mole) into the calculator to find the moles, which will be displayed in mol, mmol, µmol, nmol, and pmol.
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It’s calculated by summing the atomic masses of all atoms in the chemical formula, using the periodic table.
Use the formula \( \text{Mass (g)} = \text{Moles} \times \text{Molar Mass (g/mol)} \). However, this calculator focuses on calculating moles; for mass, you’d need to input moles and molar mass in a different configuration.