 Home
Home
 Back
Back
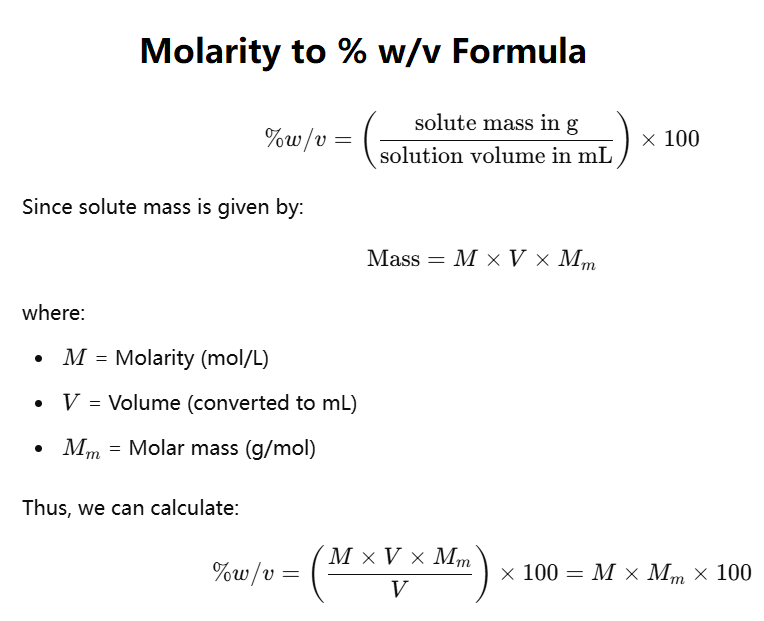
Weight/Volume % (w/v) represents the mass of solute in grams per 100 mL of solution. It is calculated as:
\%w/v = \( \frac{\text{Mass of solute (g)}}{\text{Solution volume (mL)}} \times 100 \)
Example: You prepare a solution with 500 μM NaCl in 1000 μL of water. The molar mass of NaCl is 58.44 g/mol.
First, convert molarity and volume:
500 μM = \( 500 \times 10^{-6} \) M, 1000 μL = 1 mL
Calculate mass:
Mass = \( 0.0005 \times 1 \times 58.44 \) = 0.02922 g
Then, calculate % w/v:
\%w/v = \( \frac{0.02922}{1} \times 100 \) = 2.922%