 Home
Home
 Back
Back
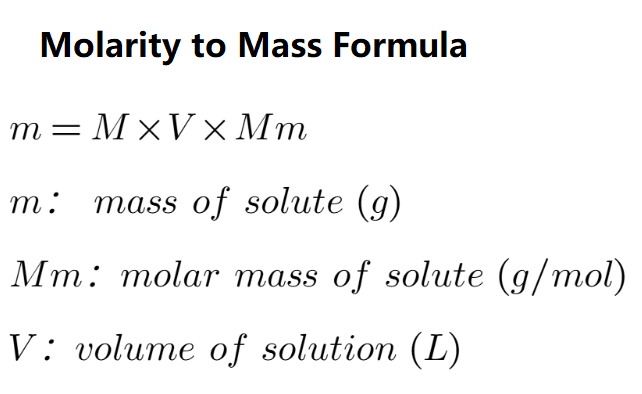
Mass (g) is the amount of solute in a solution. It is calculated using the formula:
m = \( M \times V \times M_m \)
Example: You prepare a solution with 0.5 M HCl in 250 mL of water. The formula weight of HCl is 36.46 g/mol.
Using the formula:
m = \( 0.5 \times 0.25 \times 36.46 \) = 4.557 g
Thus, you need 4.557 g of HCl to prepare this solution.