 Home
Home
 Back
Back
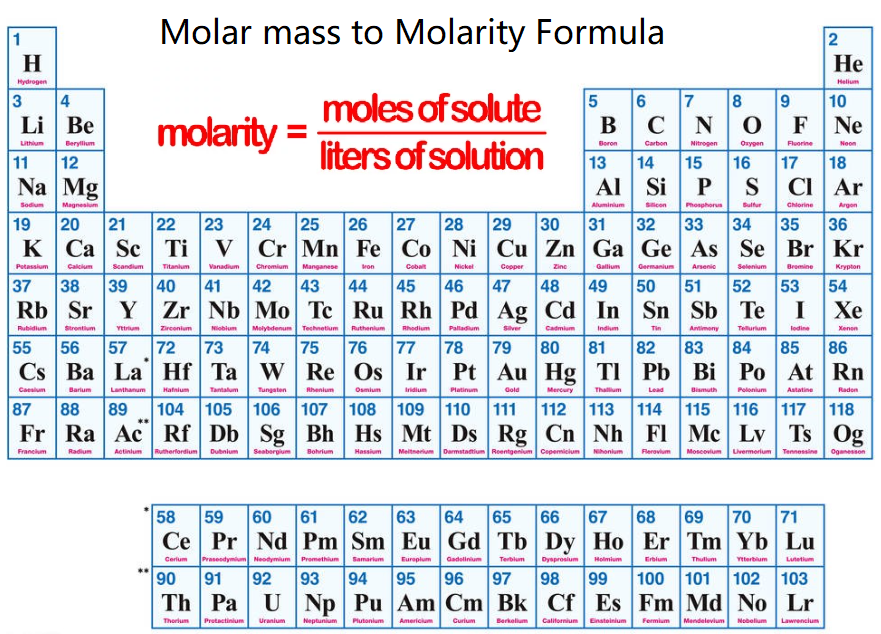
Molarity (M) is the concentration of a solution expressed as the number of moles of solute per liter of solution. It is calculated using the formula:
Molarity = \( \frac{\text{Weight (g)}}{\text{Total Molar Mass (g/mol)} \times \text{Volume (L)}} \)
Example: You dissolve 10 g of NaCl in 2 L of water. The total molar mass of NaCl is 58.44 g/mol.
Using the formula:
Molarity = \( \frac{10}{58.44 \times 2} \) = 0.0855 M
Thus, the molarity of the solution is 0.0855 M.
| Element | Symbol | Molar Mass (g/mol) |
|---|---|---|
| Hydrogen | H | 1.008 |
| Helium | He | 4.0026 |
| Lithium | Li | 6.94 |
| Beryllium | Be | 9.0122 |
| Boron | B | 10.81 |
| Carbon | C | 12.011 |
| Nitrogen | N | 14.007 |
| Oxygen | O | 15.999 |
| Fluorine | F | 18.998 |
| Neon | Ne | 20.180 |
| Sodium | Na | 22.990 |
| Magnesium | Mg | 24.305 |
| Aluminum | Al | 26.982 |
| Silicon | Si | 28.085 |
| Phosphorus | P | 30.974 |
| Sulfur | S | 32.06 |
| Chlorine | Cl | 35.45 |
| Argon | Ar | 39.948 |
| Potassium | K | 39.098 |
| Calcium | Ca | 40.078 |
| Scandium | Sc | 44.956 |
| Titanium | Ti | 47.867 |
| Vanadium | V | 50.942 |
| Chromium | Cr | 52.00 |
| Manganese | Mn | 54.938 |
| Iron | Fe | 55.845 |
| Cobalt | Co | 58.933 |
| Nickel | Ni | 58.693 |
| Copper | Cu | 63.546 |
| Zinc | Zn | 65.38 |
| Gallium | Ga | 69.723 |
| Germanium | Ge | 72.63 |
| Arsenic | As | 74.922 |
| Selenium | Se | 78.971 |
| Bromine | Br | 79.904 |
| Krypton | Kr | 83.798 |
| Rubidium | Rb | 85.468 |
| Strontium | Sr | 87.62 |
| Yttrium | Y | 88.906 |
| Zirconium | Zr | 91.224 |
| Niobium | Nb | 92.906 |
| Molybdenum | Mo | 95.95 |
| Technetium | Tc | 98 |
| Ruthenium | Ru | 101.07 |
| Rhodium | Rh | 102.91 |
| Palladium | Pd | 106.42 |
| Silver | Ag | 107.868 |
| Cadmium | Cd | 112.414 |
| Indium | In | 114.818 |
| Tin | Sn | 118.710 |
| Antimony | Sb | 121.760 |
| Tellurium | Te | 127.60 |
| Iodine | I | 126.904 |
| Xenon | Xe | 131.293 |
| Cesium | Cs | 132.905 |
| Barium | Ba | 137.33 |
| Lanthanum | La | 138.905 |
| Cerium | Ce | 140.116 |
| Praseodymium | Pr | 140.907 |
| Neodymium | Nd | 144.242 |
| Promethium | Pm | 145 |
| Samarium | Sm | 150.36 |
| Europium | Eu | 151.984 |
| Gadolinium | Gd | 157.25 |
| Terbium | Tb | 158.925 |
| Dysprosium | Dy | 162.500 |
| Holmium | Ho | 164.930 |
| Erbium | Er | 167.259 |
| Thulium | Tm | 168.934 |
| Ytterbium | Yb | 173.04 |
| Lutetium | Lu | 175.000 |
| Hafnium | Hf | 178.49 |
| Tantalum | Ta | 180.947 |
| Wolfram | W | 183.84 |
| Rhenium | Re | 186.207 |
| Osmium | Os | 190.23 |
| Iridium | Ir | 192.217 |
| Platinum | Pt | 195.084 |
| Gold | Au | 196.967 |
| Mercury | Hg | 200.592 |
| Thallium | Tl | 204.38 |
| Lead | Pb | 207.2 |
| Bismuth | Bi | 208.980 |
| Polonium | Po | 209 |
| Astatine | At | 210 |
| Radon | Rn | 222 |
| Francium | Fr | 223 |
| Radium | Ra | 226.028 |
| Actinium | Ac | 227 |
| Thorium | Th | 232.037 |
| Protactinium | Pa | 231.035 |
| Uranium | U | 238.028 |
| Neptunium | Np | 237 |
| Plutonium | Pu | 244 |
| Americium | Am | 243 |
| Curiun | Cm | 247 |
| Berkelium | Bk | 247 |
| Californium | Cf | 251 |
| Einsteinium | Es | 252 |
| Fermium | Fm | 257 |
| Mendelevium | Md | 258 |
| Nobelium | No | 259 |
| Lawrencium | Lr | 262 |
| Rutherfordium | Rf | 267 |
| Dubnium | Db | 270 |
| Seaborgium | Sg | 271 |
| Bohrium | Bh | 270 |
| Hassium | Hs | 277 |
| Meitnerium | Mt | 278 |
| Darmstadtium | Ds | 281 |
| Roentgenium | Rg | 280 |
| Copernicium | Cn | 285 |
| Flerovium | Fl | 289 |
| Moscovium | Mc | 288 |
| Livermorium | Lv | 293 |
| Tennessine | Ts | 294 |
| Oganesson | Og | 294 |