 Home
Home
 Back
Back
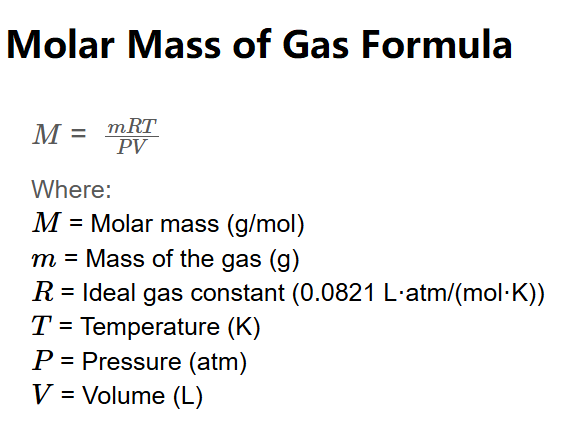
The formula for calculating the molar mass of a gas using the ideal gas law is:
\( M = \frac{mRT}{PV} \)
Where:
Moles can be calculated as \( n = \frac{m}{M} \).
Let’s say we have 1.211 g of a gas that occupies a volume of 0.677 L at a temperature of 23°C (296 K) and a pressure of 0.987 atm. The molar mass would be calculated as follows:
\( M = \frac{1.211 \times 0.0821 \times 296}{0.987 \times 0.677} = 44.0 \, \text{g/mol} \)
The moles would then be \( n = \frac{1.211}{44.0} = 0.0275 \, \text{mol} \), or 27.5 mmol, 27,500 μmol, etc., depending on the unit selected.
The molar mass of a gas is the mass of one mole of the gas, typically expressed in grams per mole (g/mol). It can be calculated using the ideal gas law if you know the mass, pressure, volume, and temperature of the gas.
Use the formula \( M = \frac{mRT}{PV} \), where \( m \) is the mass of the gas (g), \( R \) is the ideal gas constant (0.0821 L·atm/(mol·K)), \( T \) is the temperature in Kelvin, \( P \) is the pressure in atm, and \( V \) is the volume in liters. Ensure all units match the value of \( R \), or use unit conversions as provided in the calculator.
The ideal gas constant (\( R \)) is 0.0821 L·atm/(mol·K) when using pressure in atm, volume in liters, and temperature in Kelvin. It relates the energy scale to the temperature scale for an ideal gas.
You can find the number of moles (\( n \)) using the formula \( n = \frac{m}{M} \), where \( m \) is the mass of the gas (g) and \( M \) is the molar mass (g/mol) calculated from the ideal gas law. Use unit conversions if necessary, and select the desired unit (mol, mmol, μmol, nmol, pmol) from the dropdown.