 Home
Home
 Back
Back
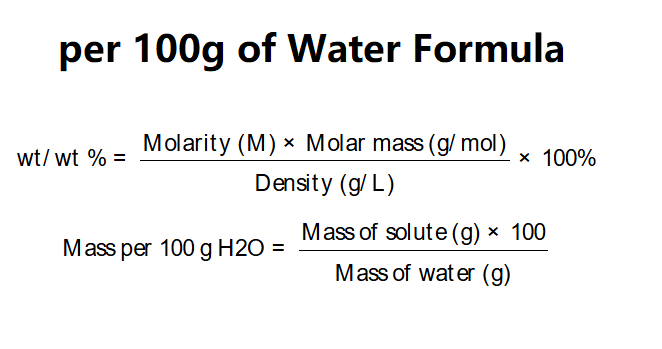
Definition: This calculator determines the percentage concentration by mass (wt/wt %) and mass of solute per 100 g of water (H2O) in a solution based on its molarity, molar mass of solute, and density.
Purpose: It is used in chemistry to express the concentration of a solution relative to water, useful for preparing solutions, analyzing chemical compositions, and understanding solubility.
The calculator uses the following formulas:
Explanation: Enter the molarity (e.g., 3 M), molar mass of solute (e.g., 58.5 g/mol for NaCl), and density of the solution (e.g., 1116 g/L). The calculator converts units, calculates wt/wt %, and determines the mass of solute per 100 g of water in various units.
Details: This measure is critical for understanding solute concentration in aqueous solutions, aiding in formulation, quality control, and scientific research involving water-based systems.
Tips: Input the molarity (e.g., 3 M), molar mass (e.g., 58.5 g/mol), and density (e.g., 1.116 g/cm³). Select the appropriate units for molarity, molar mass, and density from the dropdowns.