 Home
Home
 Back
Back
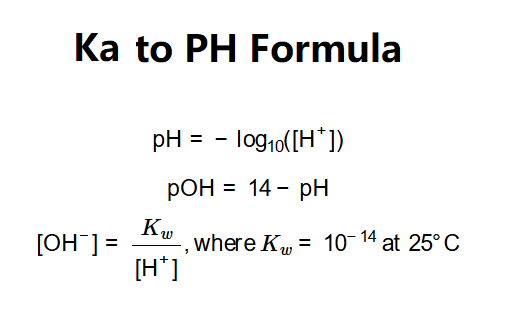
Definition: This calculator determines the pH, pOH, [H⁺], and [OH⁻] of a solution based on the Ka (acid dissociation constant) and concentration of a weak acid, with customizable units for both input and output.
Purpose: It helps chemists, students, and researchers calculate pH and ion concentrations for weak acid solutions, useful in chemistry, biology, and environmental science.
The calculator uses these principles:
Where:
Explanation: Enter a valid Ka value, input the concentration with its unit, choose the output units for [H⁺] and [OH⁻], and click "Calculate." Results show pH, pOH, [H⁺], and [OH⁻] in the chosen units, with values < 0.00001 displayed in scientific notation.
Details: These measurements are crucial for understanding acidity and basicity, biological processes, industrial applications, and environmental conditions. Accurate values help in chemical reactions, buffer design, and health assessments.
Tips: Enter a positive Ka value (e.g., 1.8e-5 for acetic acid), input a positive concentration with its unit, select the [H⁺] and [OH⁻] output units, and click "Calculate." Ensure Ka is in decimal or scientific notation (e.g., 1.8e-5). Results are displayed with scientific notation for values < 0.00001.