 Home
Home
 Back
Back
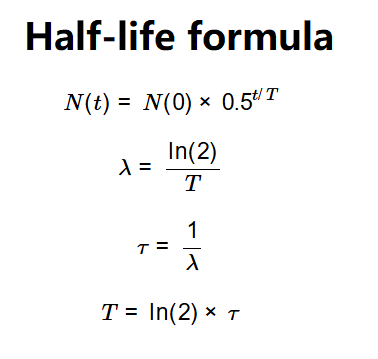
Definition: This calculator determines the remaining quantity (\(N(t)\)), decay constant (\(\lambda\)), and mean lifetime (\(\tau\)) of a radioactive or decaying substance based on its initial quantity, half-life time, and total time elapsed.
Purpose: It is used in nuclear physics, chemistry, and biology to predict the decay of radioactive isotopes, pharmaceuticals, or other unstable substances, aiding in safety, dating, and process design.
The calculator uses the following formulas:
Where:
Explanation: Enter the initial quantity (e.g., 2.5 kg), half-life (e.g., 19.88 minutes), and total time (e.g., 5 minutes). Select the output time unit for \(\lambda\) and \(\tau\) (e.g., minutes). The calculator converts times to seconds, computes \(N(t)\), \(\lambda\), and \(\tau\), and displays \(\lambda\) and \(\tau\) in all time units based on the selected output unit.
Notes on Units: Ensure \(N(0)\), \(T\), and \(t\) are positive and non-zero. Time units are converted to seconds for calculation, then \(\lambda\) and \(\tau\) are converted to all specified units (sec, min, hours, days, week, month, years). \(N(t)\) assumes the same units as \(N(0)\).
Details: Half-life calculations are essential for understanding radioactive decay, dating archaeological samples, managing nuclear waste, and predicting the stability of chemical or biological systems.
Tips: Input the initial quantity (positive number), half-life, and total time with their respective units. Select the output time unit for \(\lambda\) and \(\tau\) to view results in all time units. Ensure all values are positive and physically meaningful. Results are approximate for ideal exponential decay; adjust for non-ideal conditions if needed.