 Home
Home
 Back
Back
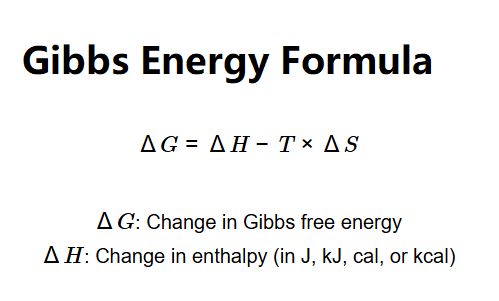
Definition: This calculator determines the Gibbs free energy change (\(\Delta G\)) for a chemical reaction based on the enthalpy change (\(\Delta H\)), entropy change (\(\Delta S\)), and temperature (\(T\)), using the Gibbs free energy formula.
Purpose: It is used in chemistry and thermodynamics to predict the spontaneity and feasibility of reactions, aiding in reaction design, process optimization, and understanding system stability.
The calculator uses the Gibbs free energy equation:
Where:
Explanation: Enter the enthalpy change (e.g., -100 kJ), entropy change (e.g., 0.1 kJ), and temperature (e.g., 25°C). The calculator converts units to joules (J) and Kelvin (K), computes \(\Delta G\) in J, and outputs it in J, kJ, cal, and kcal. Note that \(\Delta S\) should be in energy units per Kelvin (e.g., J/K) for correct calculation; if per mole, ensure consistency with \(\Delta H\).
Important Note: If \(\Delta S\) is provided in energy units (J, kJ, cal, kcal) without specifying per Kelvin, the calculator assumes it’s per mole at standard conditions (298.15 K) for simplicity. For precise calculations, ensure \(\Delta S\) is in J/K or J/mol/K, and adjust inputs accordingly.
Details: Gibbs free energy determines reaction spontaneity (\(\Delta G < 0\) for spontaneous reactions), combining enthalpy and entropy effects. It’s crucial for predicting equilibrium, reaction direction, and thermodynamic feasibility in chemical and biological systems.
Tips: Input \(\Delta H\), \(\Delta S\), and \(T\) with their respective units. Ensure \(\Delta S\) is in energy units per Kelvin (e.g., J/K) for correct units in \(\Delta G\). All values should be positive or negative as appropriate for the reaction. Results are approximate if \(\Delta S\) units are not explicitly per Kelvin; adjust for per mole if using standard entropy values.