 Home
Home
 Back
Back
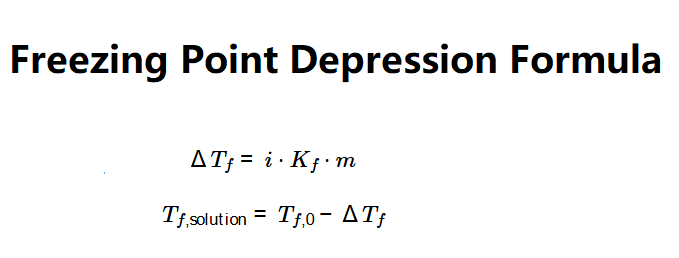
Definition: This calculator determines the freezing point depression (\(\Delta T_f\)) and the freezing point of a solution (\(T_{f,\text{solution}}\)) based on the molality of the solution, an optional solvent selection (with predefined freezing point depression constants and freezing points), and an optional van’t Hoff factor.
Purpose: It is used in chemistry to predict how the freezing point of a solvent changes when a solute is added, aiding in solution preparation, colligative property studies, and industrial applications like antifreeze solutions and road salting.
The calculator uses the freezing point depression formula:
Where:
Explanation: Enter the molality (e.g., 0.4 mol/kg for ethylene glycol in water), optionally select a solvent (e.g., water, which autofills \(K_f = 1.86 \, \text{°C·kg/mol}\) and freezing point \(0.0 \, \text{°C}\)), and optionally input the van’t Hoff factor (defaults to 1). The calculator computes \(\Delta T_f\) and \(T_{f,\text{solution}}\) in °C, °F, and K, displaying them in separate sections. If a solvent is selected, custom \(K_f\) and freezing point fields are hidden; select "None (Custom)" to enter manual values.
Van’t Hoff Factor: For nonelectrolytes (e.g., sugar), \(i = 1\). For electrolytes (e.g., NaCl, \(i \approx 1.9\)), \(i\) accounts for dissociation, increasing the effective number of particles in solution.
Details: Freezing point depression is a colligative property, critical for applications like antifreeze in car radiators, road salting in winter, and ice cream production, where lowering the freezing point prevents freezing or creates desirable textures.
Tips: Input the molality with its unit (mol/kg or mmol/kg), optionally select a solvent to autofill \(K_f\) and freezing point, or choose "None (Custom)" to manually enter \(K_f\) and freezing point. Optionally input the van’t Hoff factor (defaults to 1); use values >1 for electrolytes (e.g., 1.9 for NaCl). Ensure all values are positive, and \(K_f\) is appropriate for the solvent. Results are approximate for ideal solutions.