 Home
Home
 Back
Back
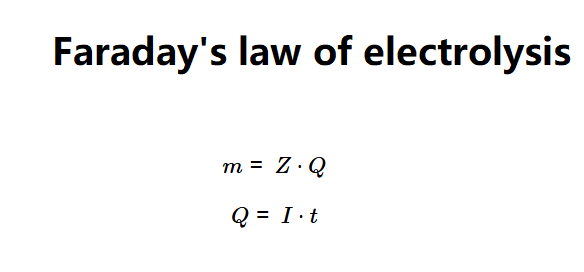
Definition: This calculator determines the time (\(t\)) and mass (\(m\)) involved in an electrolysis reaction based on the electrochemical constant (\(Z\)), charge (\(Q\)), or current (\(I\)), using Faraday's law of electrolysis.
Purpose: It is used in chemistry and electrochemistry to predict mass changes and time durations in electrolytic cells, aiding in industrial processes, battery design, and experimental analysis.
The calculator uses Faraday's law of electrolysis:
Where:
Explanation: Select a substance (e.g., Copper) or choose "Custom" to input \(Z\) (e.g., 3.295e-7 kg/C for Cu). Enter charge (e.g., 0.1 A · 60 s = 6 C) or current (e.g., 0.1 A) and optionally time. The calculator converts units, computes mass using \(m = Z \cdot Q\), and time using \(t = Q / I\) if needed, outputting results in multiple units.
Notes on Direction:** The mass calculation assumes the direction of the reaction (deposition or dissolution) based on the electrochemical constant and charge flow. Positive mass indicates deposition (e.g., Cu ions to electrode), negative indicates dissolution (e.g., Zn from electrode), but the calculator outputs absolute values with context in the explanation.
Details: Electrolysis calculations are essential for understanding and optimizing electrochemical processes, such as metal plating, battery operation, and water splitting, impacting industrial, environmental, and energy applications.
Tips: Select a substance or input a custom \(Z\) (in kg/C). Provide either charge (in µC, mC, C, mAh, Ah) or current (in µA, mA, A) with optional time. Ensure all values are positive and appropriate for the reaction. Results are approximate for ideal conditions; adjust for non-ideal electrolytes or incomplete reactions.