 Home
Home
 Back
Back
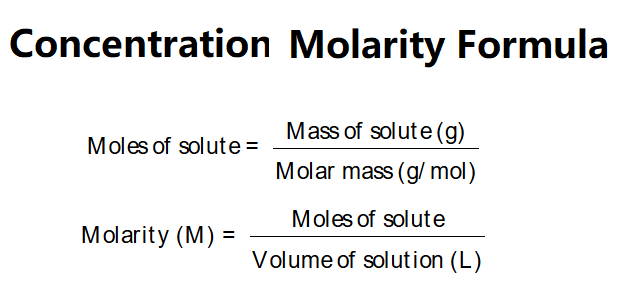
Definition: This calculator determines the moles of solute and the molarity (concentration in mol/L) of a solution based on the mass of solute, its molar mass, and the volume of the solution.
Purpose: It is used in chemistry to calculate the concentration of a solution in molar units, essential for preparing solutions, conducting experiments, and understanding chemical reactions.
The calculator uses the following steps:
Explanation: Enter the mass of solute (e.g., in mg, g, kg, lb, or oz), the molar mass of solute (e.g., in g/mol, kg/mol, etc.), and the volume of solution (e.g., in cubic centimeters, liters, etc.). The calculator converts units, calculates moles, and determines molarity in various units (M, mM, μM, nM, pM, fM).
Details: Molarity is a key measure of concentration in chemistry, used to describe the number of moles of solute per liter of solution, crucial for stoichiometry, titration, and solution preparation.
Tips: Input the mass of solute (e.g., 5 g), molar mass (e.g., 58.44 g/mol for NaCl), and volume of solution (e.g., 1 L). Select the appropriate units for mass, molar mass, and volume from the dropdowns.