 Home
Home
 Back
Back
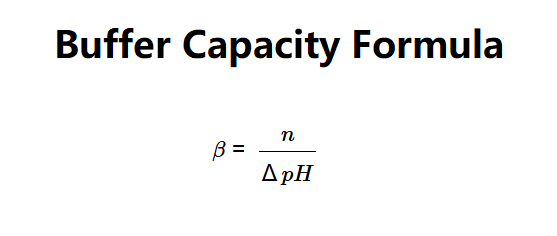
Definition: This calculator determines the buffer capacity of a solution based on the amount of acid or base added per unit volume and the change in pH.
Purpose: It helps assess a buffer solution's ability to resist pH changes, critical for applications in chemistry, biology, and pharmaceuticals.
The calculator uses the formula:
Where:
Explanation: Enter the amount of acid/base (in moles per unit volume), initial pH, and final pH. Select the appropriate unit for the amount, and the calculator converts and computes buffer capacity in all specified units.
Details: Buffer capacity indicates how well a solution maintains a stable pH, essential for biochemical processes, industrial applications, and laboratory experiments.
Tips: Input the amount of acid/base (e.g., 0.001 mol/L), initial pH (e.g., 7.0), and final pH (e.g., 7.2). Choose the unit (e.g., mol/liter) from the dropdown, and the calculator will display buffer capacity in all listed units.