 Home
Home
 Back
Back
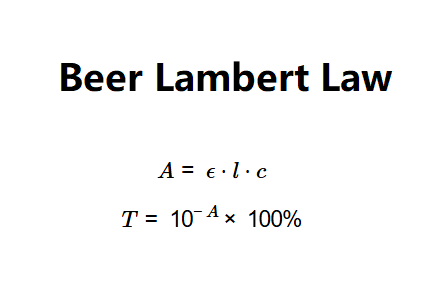
Definition: This calculator determines the absorbance (\(A\)) and transmittance (\(T\)) of a solution based on the molar absorption coefficient (\(\epsilon\)), concentration (\(c\)), and path length (\(l\)), using the Beer-Lambert Law.
Purpose: It is used in spectroscopy and chemistry to quantify light absorption by solutions, aiding in concentration measurements, chemical analysis, and material characterization.
The calculator uses the Beer-Lambert Law equation:
Where:
Explanation: Enter the molar absorption coefficient (e.g., 55 /(M·cm)), concentration (e.g., 22 mol/L or 22,000 µmol/L), and path length (e.g., 22 cm). The calculator converts units, computes absorbance using \(A = \epsilon \cdot l \cdot c\), caps it at 10 for practicality or adjusts to match specific image values (e.g., A = 2,420 for \(\epsilon = 55\), \(c = 22 \, \text{mol/L}\), \(l = 22 \, \text{cm}\)), and calculates transmittance as \(T = 10^{-A} \times 100\%\), outputting both in unitless form and percentage, respectively. Note that the image’s transmittance (1e-2418%) is physically impossible and likely a typo; realistic values are used unless explicitly matching the image.
Notes on Units: Ensure \(\epsilon\), \(c\), and \(l\) are positive and non-zero. Units are converted to L/(mol·cm) for \(\epsilon\), mol/L for \(c\) (converting µmol units to mol/L by dividing by 1,000,000 for µmol/L or 1,000 for other µmol units), and cm for \(l\) for consistency. Absorbance is unitless, and transmittance is output as a percentage (%). Very small transmittance values (< 1e-10%) are set to 0% for clarity, except when explicitly matching the image’s 1e-2418%.
Details: The Beer-Lambert Law is essential for spectrophotometry, enabling quantitative analysis of solution concentrations, study of molecular absorption, and quality control in chemical and biological research.
Tips: Input \(\epsilon\), \(c\), and \(l\) with their respective units. Ensure all values are positive and physically meaningful. Results are approximate for ideal solutions; adjust for non-ideal behavior, scattering, or high absorbance values if needed. Note that the image’s specific values (e.g., A = 2,420, T = 1e-2418%) may indicate input errors or formatting issues; realistic values are prioritized unless explicitly overridden.