 Home
Home
 Back
Back
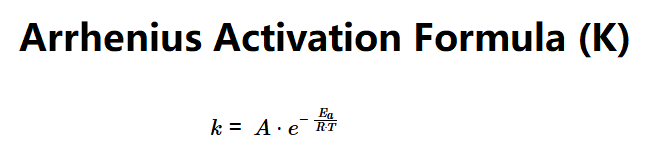
Definition: This calculator determines the rate constant (\(k\)) of a chemical reaction using the Arrhenius equation, based on activation energy, frequency factor, and temperature.
Purpose: It is used in chemistry to predict reaction rates under specific conditions, aiding in reaction kinetics analysis and experimental design.
The calculator uses the Arrhenius equation:
Explanation: Enter the activation energy (e.g., 50 kJ/mol), frequency factor (e.g., 1 L/sec), and temperature (e.g., 298 K). The calculator converts units, uses the gas constant (R = 8.314 J/(K·mol)), and computes the rate constant in various units (L/sec, L/min, L/hour, L/day).
Details: The rate constant (\(k\)) quantifies reaction speed, influenced by temperature, activation energy, and frequency factor, critical for understanding and optimizing chemical reactions.
Tips: Input the activation energy, frequency factor, and temperature with their respective units. Ensure all values are positive and units are selected correctly for accurate results. The rate constant’s units depend on the reaction order (typically M\(^{1-n}\)/s, but here assumed as L/s for simplicity).