 Home
Home
 Back
Back
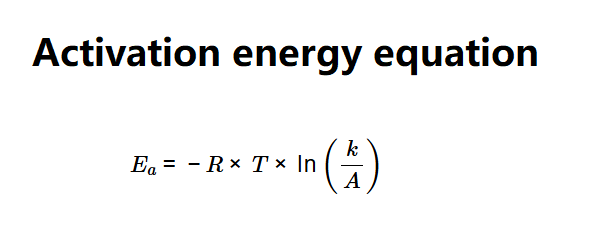
Definition: This calculator determines the activation energy (Ea) of a chemical reaction using the Arrhenius equation, based on temperature, reaction rate coefficient, and frequency factor.
Purpose: It is used in chemistry to understand the energy barrier a reaction must overcome, aiding in reaction rate analysis and experimental design.
The calculator applies the Arrhenius equation:
Explanation: Enter the temperature (e.g., 298 K), reaction rate coefficient (e.g., 0.1 L/sec), and frequency factor (e.g., 1 L/sec). The calculator converts units, uses the gas constant (R = 8.314 J/(K·mol)), and computes activation energy in various units (J/mol, kJ/mol, MJ/mol, cal/mol, kcal/mol).
Details: Activation energy indicates the minimum energy required for a reaction to proceed, influencing reaction rates, temperature dependence, and catalyst effectiveness in chemical processes.
Tips: Input the temperature (e.g., 25°C), reaction rate coefficient, and frequency factor with their respective units. Ensure all values are positive and units are selected correctly for accurate results. Note that the frequency factor and reaction rate must have compatible units (e.g., both in L/sec).